Se Cation Or Anion
Posted : admin On 7/22/2022- Se Cation Or Anion Chemical
- Examples Of Anions And Cations
- Is Se A Cation Or Anion
- List Of Cations And Anions
- Se Cation Or Anion Definition
nombre
Se Cation Or Anion Chemical
It reflects the difference between the positively charged ions (called cations) and the negatively charged ions (called anions). An abnormal anion gap is non-specific—it does not diagnose a specific disease or illness—but it can suggest certain kinds of metabolic or respiratory disorders or the presence of toxic substances. Cation Formula Cation Name Anion Formula Anion Name H+ Hydrogen BO 3-3 Borate Li+ Lithium CO 3-2 Carbonate Na+ Sodium HCO 3-Bicarbonate K+ Potassium NO 2-Nitrite Be+2 Beryllium NO 3-Nitrate Mg+2 Magnesium PO 2-3 Hypophosphite Ca+2 Calcium PO 3-3 Phosphite Sr+2 Strontium PO 4-3 Phosphate Ba+2 Barium HPO 4-2 Biphosphate B+3 Boron H 2PO4-Hydrogen.
Química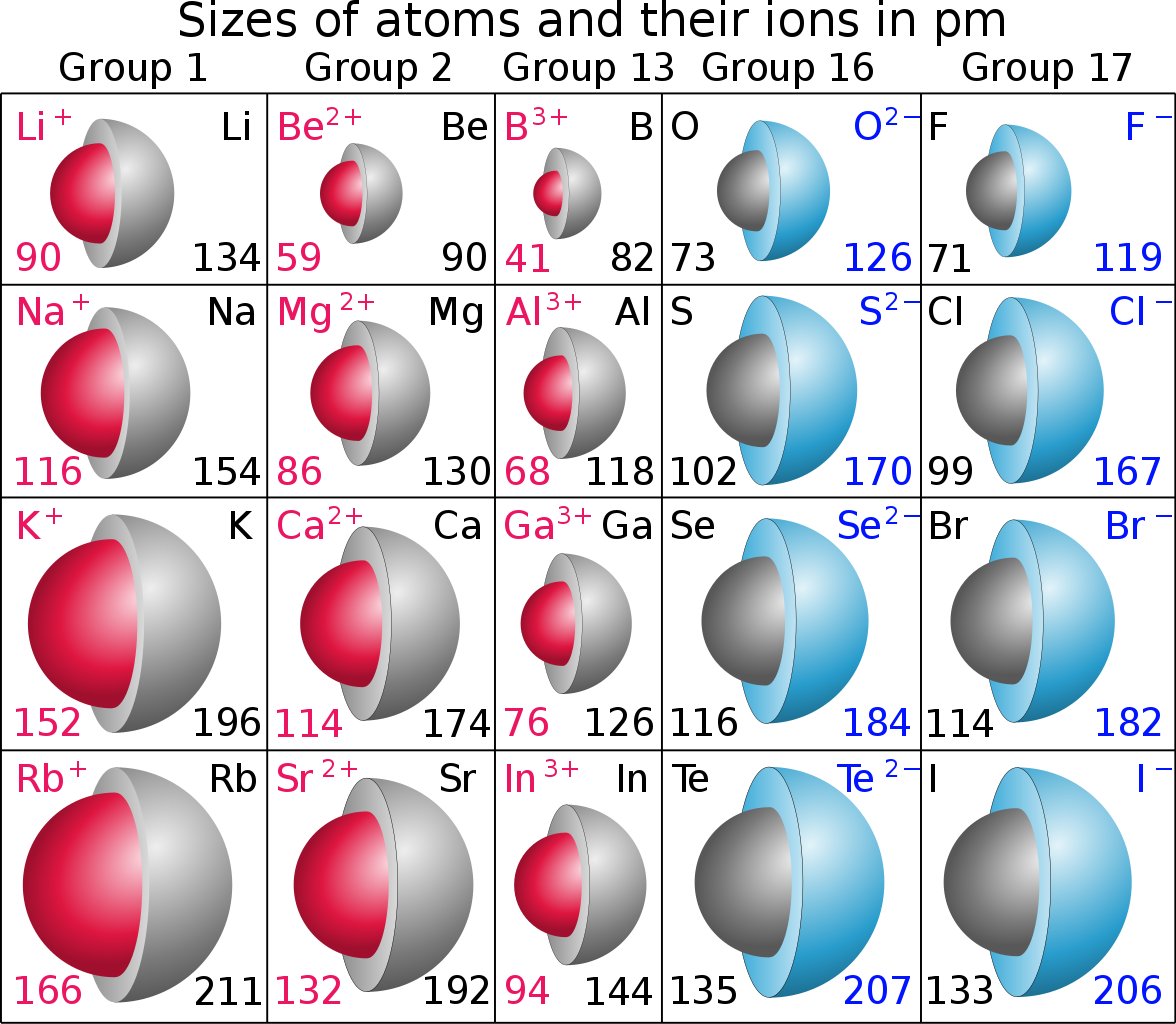
A negatively charged ion, i.e. one that would be attracted to the anode in electrolysis.
‘When two atoms swap electrons to produce a cation and an anion, the two ions are attracted to each other.’- ‘During electrolysis an anion is attracted to the anode (positive electrode).’
- ‘In an ionic compound there are two different types of ions present, the positively charged cations and the negatively charged anions.’
- ‘It is called a cation if a positive charge exists and an anion if a negative charge exists.’
- ‘It's easy to make a high oxidation state in an anion because an anion is electron-rich.’
- ‘All the positively charged ions, or cations, are together on the right side of the collage, and all the negatively charged ions, or anions, are on the left.’
- ‘An ionic compound is composed of a network of ions that results in a three-dimensional matrix of cations and anions.’
- ‘For ions dissolved in water, the rapid exchange of water molecules around the cation usually provides the space for an anion to approach the cation.’
- ‘The cation is positively charged, and the anion is negatively charged.’
- ‘Non-metals accept electrons in forming anions while metals donate electrons to form cations.’
- ‘These water molecules are ripped apart and change into hydroxyl anions, each of which is negatively charged and has one oxygen ion with a proton attached.’
- ‘The molecules or anions attached to the central atom are called coordinating groups or ligands.’
- ‘Cations within the middle solution are attracted to one electrode and anions to the other.’
- ‘A typical reaction of such compounds is to accept an additional electron from an anion or to share electrons with an anion to gain a stable octet.’
- ‘Nitric oxide can react with superoxide anions to produce peroxynitrite anions, thus quenching the biological effects of NO.’
- ‘To balance the influx of positive charge, organic anions, principally malate, and chloride are also accumulated.’
- ‘Crystals of table salt consist of equal numbers of sodium cations and chlorine anions, cation-anion pairs being held together by a force of attraction.’
- ‘Cations and anions can only exist in ionic compounds, nearly all of which are solids at room temperature, or in solution.’
- ‘The functional groups of iron hydroxides may sequestrate some cations and anions.’
- ‘Protons diffuse across a membrane from the anode chamber to the cathode chamber, where they react with the anions to form water or ferrocyanide ions.’
Origen
The difference between cation and anion is that cation contains a positive charge on it while an anion exhibit a negative charge on it.
Cation and anion are atoms having a charge on them. Here we will come to know the differences between both of these charged particles. Cations exhibit a positive charge on them while anion exhibit a negative charge.
The word ‘cation’ is taken from the Greek word “ kata “ which means down. While the word anion is originated from the Greek word “ano” which means up. Cations are always attracted towards the cathode which is a negatively charged electrode while anions are always attracted towards the anode which is a positively charged electrode.
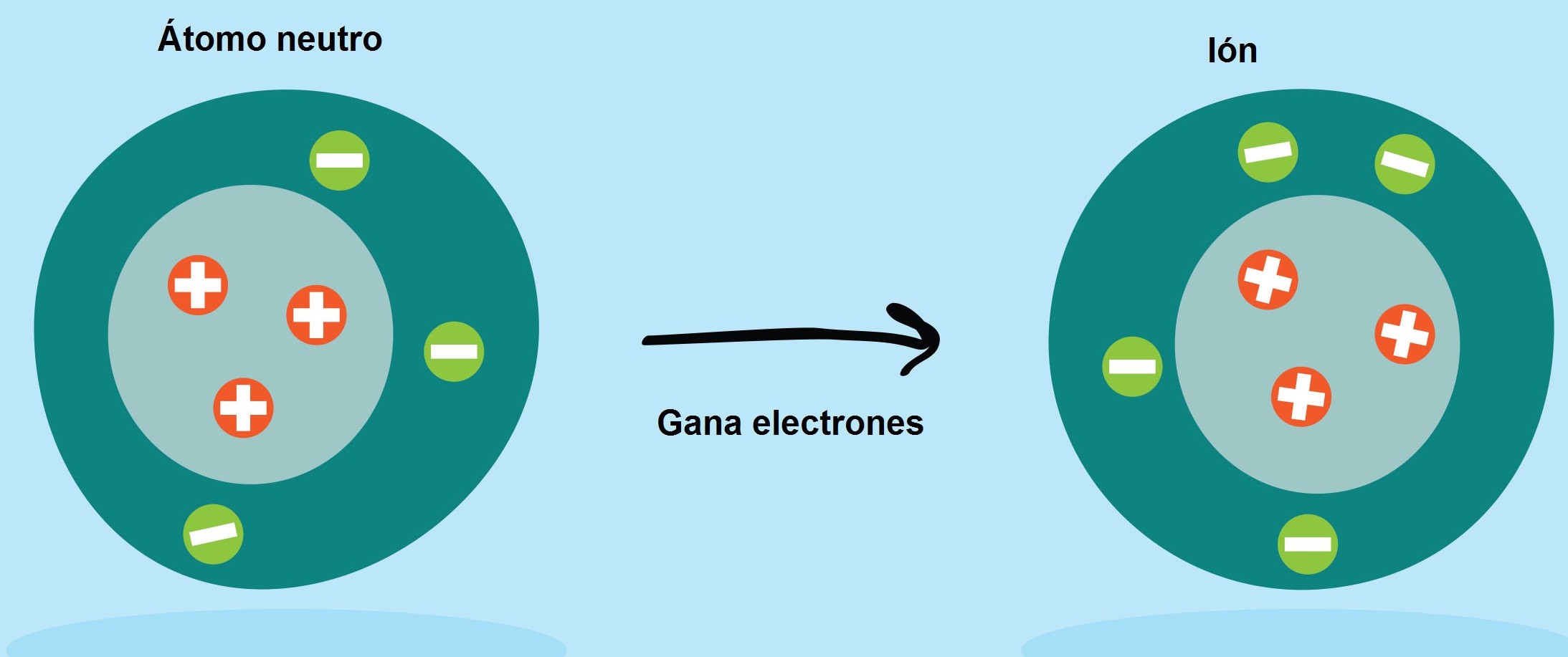
Cations are formed when an atom loses its one or more electrons, and hence positive charge appears on the atom which becomes cation then. An anion is formed when an atom gains one or more electrons and thus become negatively charged. Cations are formed from the atoms of metals while anions are formed from the elements of nonmetals. In cations, the number of protons is more than the number of electrons, and that is why it shows a positive charge. In the anion, the number of electrons is greater than protons, and that is why they exhibit negative charge.
In a chemical reaction, cations react with anions to form an ionic compound. A cation can never react with a cation, and an anion can never react with an anion. The radius of a cation is always greater than the radius of an anion because, in cations, orbits are lost with the loss of electrons while in anions, orbit number is increased with the gain of electrons. A cation which exhibits a positive charge is denoted with a superscript + after the name of the element or chemical formula of a compound, e.g., Fe2+ and NH4+. An anion is also denoted in the same way with a superscript of – after the name of the element or chemical formula of a compound, e.g., Br- N3- etc. The examples of cations are iron (Fe2+ and Fe3+), Sodium (Na+), potassium (K+) and magnesium (Mg2+) . The examples of anions are chloride (Cl-), Flouride (F-), bromide (Br-), hydride (H-) and nitride (N-).
Comparison Chart

| Basis | Cation | Anion |
| Definition | They are the type of particles which exhibit positive charge. | They are the type of particles which exhibit negative charge. |
| Why they possess charge ? | They possess a positive charge because they lose one or more electrons. | They possess a negative charge because they gain one or more electrons. |
| Origination of words | The word cation is originated from the Greek word “kata” which means down. | The word anion is originated from the Greek word “ano” which means down. |
| Electron to proton ratio | They exhibit a positive charge because the number of protons is greater than the number of electrons in them | They exhibit a negative charge because the number of the electron is greater than the number of proton in them. |
| Type of atoms | They are formed from the atoms of metals | They are formed from the atoms of nonmetals. |
| Attraction towards electrodes | They are attracted towards the negatively charged electrode, i.e. cathode. | They are attracted towards the positively charged electrode, i.e. anode. |
| How they are denoted | They are denoted by the superscript + after the name of the element or chemical formula of the compound. | They are denoted by the superscript – after the name of the element or chemical formula of the compound. |
| Radius | The radius of cations is smaller because the number of orbits becomes less due to the loss of electrons | The radius of anions is larger than the radius of cations because the number of orbits is increased with the gain of electrons. |
| Chemical reaction | In a chemical reaction, they react with anions to form ionic compounds | In a chemical reaction, they react with cations to form ionic compounds. |
| Do not react with | They do not attract or react with positively charged particles | They do not attract or react with positively charged particles |
| Examples | The examples of cations can be given as Iron (Fe2+ and Fe 3+), calcium (Ca2+), potassium (K+), aluminium Al3+) and ammonium ion (NH4+) etc. | The examples of anions can be given as bromide (Br-), chloride (Cl-) , nitride (N-) and hydride (H-), etc. |
What are cations?
Examples Of Anions And Cations
Cations are positively charged particles. They are formed when an atom of an element loses one or more electrons. Atoms do so to gain stability. In other words, they want to get the order of noble gases which are the most stable elements in the universe. The word cation is originated from the Greek word “kata” which means down. In a cation, the number of protons is greater than that of electrons. As we know, protons possess a positive charge and electrons possess a negative charge. Due to the higher number of positively charged particles, cations show positive charge. Cations are always formed from the atoms metals. The reason being is that metals have a tendency to lose electrons. A metallic surface has a countless number of free electrons on it. Thus metals lose electrons, and their atoms exist in the form of cations.
Cations are always attracted towards cathode that is a negative electrode. In a chemical reaction, cations always react with anions to form ionic compounds. One best example of this type of reaction is the formation of common salt, i.e., sodium chloride (NaCl) in which sodium is a cation and chloride is an anion. The examples of cations can be given as sodium (Na+), potassium (K+), lithium (Li+), magnesium (Mg2+) and aluminum (Al3+). An example among positively charged compounds is of ammonium ion (NH4+).

What are anions?
Is Se A Cation Or Anion

Anions are the atoms which possess a negative charge. They exhibit a negative charge because the atoms gain one or more electrons to gain stability. Thus the number of electrons becomes greater than the number of protons in those atoms, and they show a negative charge. Mostly the atoms of nonmetals show this tendency. They exhibit this type of behavior in order to follow the noble gases which are the most stable element of this universe.
Anions are always attracted towards the positive electrode, the, i.e. anode. In a chemical reaction, anions react with cations to form ionic compounds. The word anion is originated from the Greek word “ano” which means up. The examples of anions can be given as sulfur (S-), Iodide ion (I-), bromide (Br-), chloride (Cl-), hydride (H-) and nitride (N-).
Key Differences between Cation and Anion
- Cations are those particles which possess positive charge while anions are those particles which possess a negative charge.
- Cations have a tendency to move towards a negative electrode, i.e., cathode while anions have a tendency to move towards the positive electrode, i.e., the anode.
- In cations, protons are more in number than electrons while in anions, electrons are more in number than protons.
- Cations are formed by the atoms of metallic elements while anions are formed from the atoms of nonmetallic elements.
- The orbits of anions are larger than the orbits of cations.
Conclusion
List Of Cations And Anions
Cations and anions, both are charged particles. It is very important for science students to know the differences between both of them. In the above article, we learned clear differences between cations and anions.